NEOBONE GELTM
Injectable bone substitute
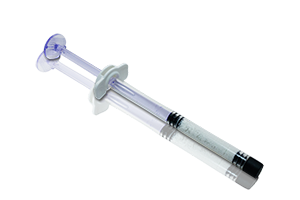
TECHNICAL FEATURES
Developed to facilitate handling during bone grafting procedures, Neobone Gel can fit into any grafting site.
Bioactive
Osteoconductive
70% porosity: interconnected network of macropores and micropores
Optimal balance between microporous MBCP® granules and the hydrogel which acts as a carrier for rapid vascularization and mineralization
More than 30 years of clinical experience (MBCP® Technology)
Not FDA approved
Manufacturer: Biomatlante SA, 5 rue Edouard Belin, ZA Les Quatre Nations – 44360 Vigneux de Bretagne – France. Neobone Gel™ (classe III). Bone substitute. Filling substitute of skeletal system of failing bones. Read carefully the instructions on the instructions for use and the surgical technique. Notified body : TÜV SÜD Product Service GmbH n°0123. Source : www.biomatlante.com – onglet REFERENCES

