SQUALETM
Anterior
cervical cage
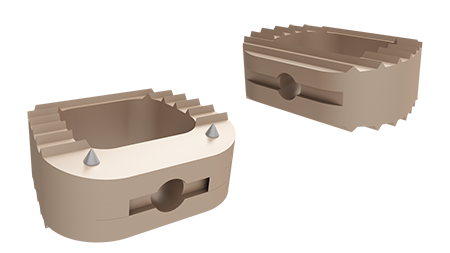
Technical features
Neobone™: Bone substitute for Squale™ cervical cages
- Composition: 60% Hydroxyapatite, 40% Beta tricalcium phosphate (β-TCP)
- Bone substitute´s shape adapted to the cage
- Packaged per one and sterile

Not FDA approved
Manufacturer: Orthopaedic & Spine Development (OSD). SQUALE™ (class IIb). Medical device for cervical arthrodesis. Read carefully the instructions on the instructions for use and the surgical technique. Notified Body: G-MED n°0459
Manufacturer: Biomatlante SA, 5 rue Edouard Belin, ZA Les Quatre Nations – 44360 Vigneux de Bretagne – France. Synthetic Bone Substitute for cage SQUALE™ (classe III). Filling substitute of skeletal system of failing bones. Read carefully the instructions on the instructions for use and the surgical technique. Notified body : TÜV SÜD Product Service GmbH n°0123.

